The Atomic Model
Dalton
The idea of an atom was first proposed by John Dalton in 1803, who realized that all matter was made up of smaller particles or units. He believed that atoms were small, dense “billiard balls.”
Thomson
J.J. Thomson decided to test this idea of an atom in 1897. He used a cathode ray tube, which looked similar to a soda bottle, and placed two pieces of metal into the tube. The metal discs were then attached to a power source, making one positive and the other negative. This caused a thin ray to shoot out from the discs. Thomson then surrounded the tube with a positively charged metal and negatively charged metal, and watched as the ray turned upward toward the positive metal. He also tried it with a magnet, which confirmed his findings: the ray had a negative charge. Since “opposites attract,” the positive metal forced the negative ray upward. Therefore, J.J. Thomson discovered electrons, or subatomic particles with a negative charge.
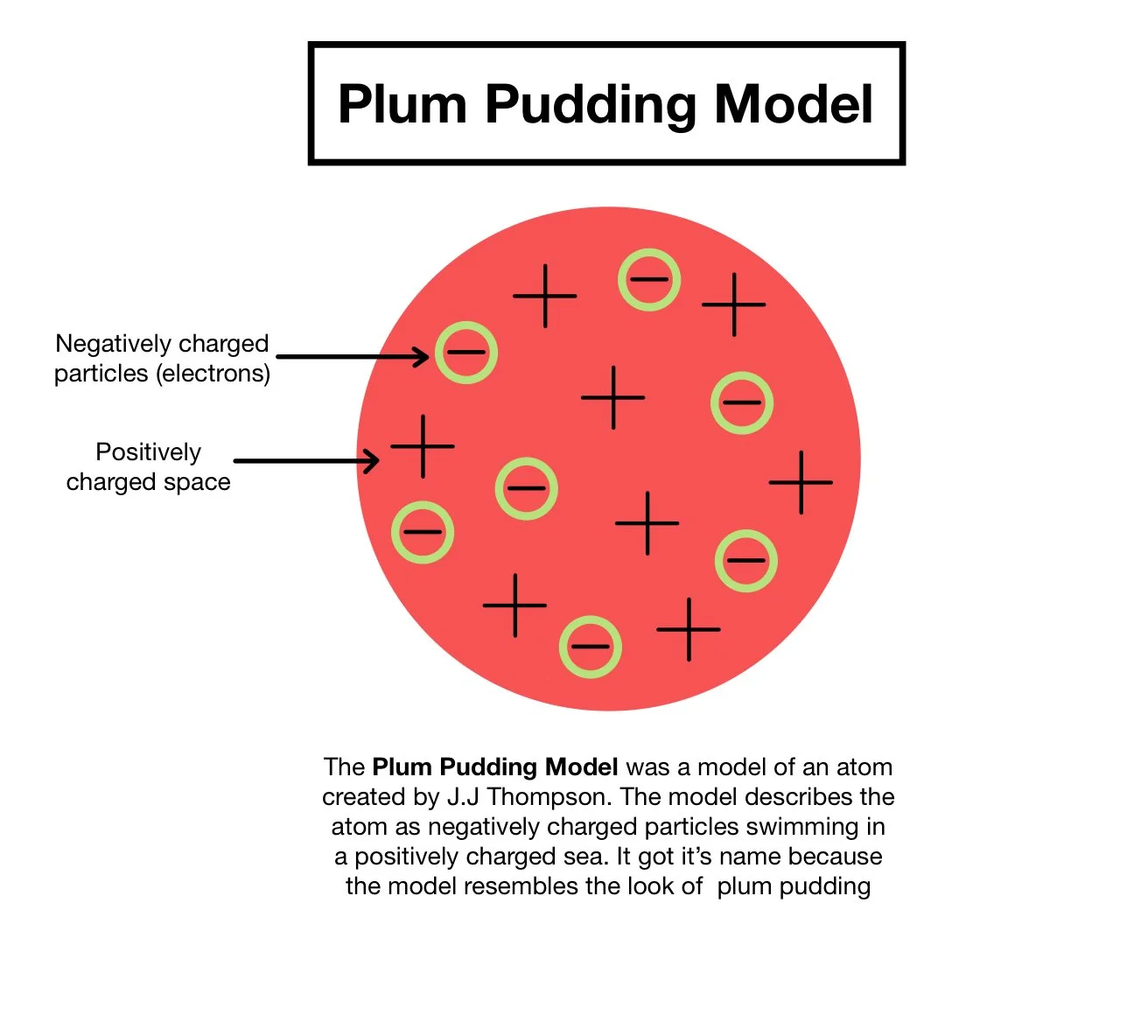
Similarly, he realized that there must be a positive charge within the atom, since atoms are generally neutral. So he created the “plum pudding model,” or, for Americans, the chocolate chip cookie..
Image Source: Caroline Monahan
Rutherford
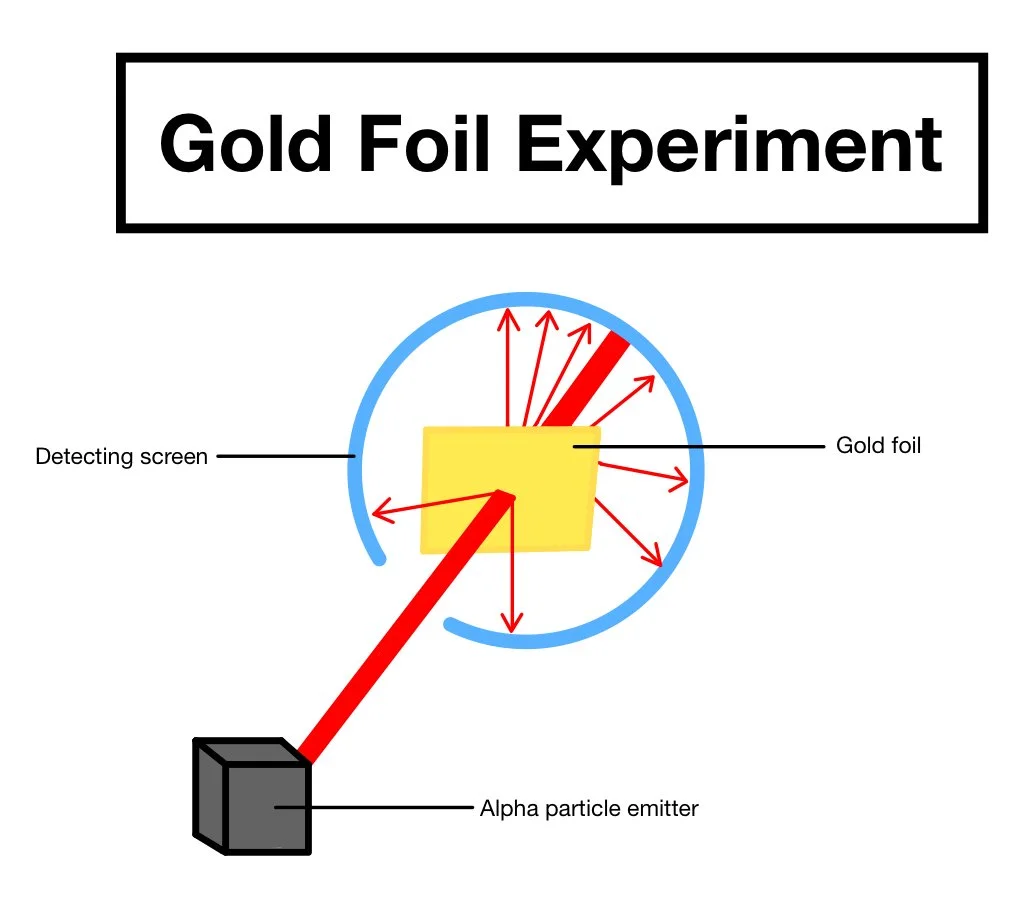
Ernest Rutherford expanded on this idea by using alpha particles, positive particles, and shooting them at a thin piece of gold foil in 1911. Based on Thomson’s model, he expected the alpha particles to go straight through the gold foil, which most of them did, but some wound up more than 90 degrees from the foil.
Image Source: Caroline Monahan
From this experiment, Rutherford could conclude that: 1) the atom is mostly empty space, since it can pass through the gold foil so easily, and 2) the atom has a dense and positively charged center. He believed that the alpha particles bounced off the gold foil’s atoms because, every so often, a particle's center would collide with a gold foil atom’s center. Similarly, since alpha particles are positive, he thought that those centers were positively charged, causing them to repel or not be attracted to one another and force each other away.